メラノスタチン
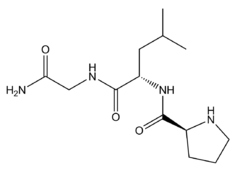
メラノスタチン(Melanostatin)は、オキシトシンの切断により生じる内在性ペプチド断片であるが、体内でオキシトシンとは異なる活性を示す[1][2]。Pro-Leu-Gly-NH2の配列のトリペプチドである。Melanocyte-inhibiting factor(MIF-1)とも言う。オピオイド受容体の活性化の影響を阻害する[3][4][5][6][7][8]と同時にドーパミン受容体D2及びD4の正のアロステリック調節因子となり[9][10][11][12][13][14][15][16][17]、またαメラニン細胞刺激ホルモン[18][19][20]等の他の神経ペプチドの放出阻害、メラトニン活性の増強などの活性[21]により、様々な効果を示す。
 | |
| IUPAC命名法による物質名 | |
|---|---|
| |
| 臨床データ | |
| MedlinePlus | a605038 |
| 薬物動態データ | |
| 生物学的利用能 | 100% (injected) |
| 代謝 | plasma protease enzymes |
| 排泄 | N/A |
| データベースID | |
| CAS番号 |
2002-44-0 |
| PubChem | CID: 92910 |
| ChemSpider |
83871 |
| UNII |
3KY24B4Q62 |
| 化学的データ | |
| 化学式 | C13H24N4O3 |
| 分子量 | 284.355 g/mol |
| |
メラノスタチンの投与は、この複雑な作用の組み合わせによって、抗うつ薬[22][23][24]、スマートドラッグ[25][26][27][28]、抗パーキンソン病薬としての効果を示し[29][30][31]、様々な医療用途で研究が行われている。血中での代謝に耐性を持ち[32]、血液脳関門を容易に通過するが[33][34]、経口では効果が弱く、注射で投与される。重要な作用を持つ、密接に関連した他のペプチドとしては、Tyr-MIF-1やエンドモルフィン-1及び-2等がある[35][36][37][38][39]。
出典
編集- ^ “Regulation of formation and proposed structure of the factor inhibiting the release of melanocyte-stimulating hormone”. Proceedings of the National Academy of Sciences of the United States of America 68 (7): 1428-33. (July 1971). Bibcode: 1971PNAS...68.1428C. doi:10.1073/pnas.68.7.1428. PMC 389210. PMID 5283931.
- ^ “Prolyl-leucyl-glycinamide shares some effects with oxytocin but decreases oxytocin levels”. Physiology & Behavior 83 (3): 475-81. (December 2004). doi:10.1016/j.physbeh.2004.08.034. PMID 15581670.
- ^ “Antagonism of morphine-induced catalepsy by L-prolyl-L-leucyl-glycinamide”. European Journal of Pharmacology 53 (2): 119-25. (January 1979). doi:10.1016/0014-2999(79)90156-0. PMID 32058.
- ^ “Opiate receptor antagonism by L-prolyl-L-leucyl-glycinamide, MIF-I”. Peptides 1 (4): 293-9. (1980). doi:10.1016/0196-9781(80)90006-6. PMID 6117839.
- ^ “Effect of prolyl-leucyl-glycinamide and alpha-melanocyte-stimulating hormone on levorphanol-induced analgesia, tolerance and dependence”. Life Sciences 34 (26): 2559-66. (June 1984). doi:10.1016/0024-3205(84)90041-9. PMID 6146083.
- ^ “Antagonism of morphine analgesia by prolyl-leucyl-glycinamide (MIF-1) in humans”. Pharmacology Biochemistry and Behavior 21 (6): 975-8. (December 1984). doi:10.1016/S0091-3057(84)80083-0. PMID 6151672.
- ^ “Existence of antiopiate systems as illustrated by MIF-1/Tyr-MIF-1”. Life Sciences 39 (23): 2153-9. (December 1986). doi:10.1016/0024-3205(86)90391-7. PMID 2878336.
- ^ “Antiopioid properties of the TYR-MIF-1 family”. Methods and Findings in Experimental and Clinical Pharmacology 26 (9): 673-7. (November 2004). doi:10.1358/mf.2004.26.9.872564. PMID 15632952.
- ^ “Effects of L-prolyl-L-leucyl-glycine amide (MIF-I) on dopaminergic neurons”. Pharmacology Biochemistry and Behavior 5 (Suppl 1): 125-7. (1976). doi:10.1016/0091-3057(76)90340-3. PMID 13412.
- ^ “MIF-1: effects on norepinephrine, dopamine and serotonin metabolism in certain discrete brain regions”. Pharmacology Biochemistry and Behavior 16 (2): 229-33. (February 1982). doi:10.1016/0091-3057(82)90153-8. PMID 6122214.
- ^ “Mesolimbic and striatal dopamine receptor supersensitivity: prophylactic and reversal effects of L-prolyl-L-leucyl-glycinamide (PLG)”. Peptides 6 (2): 179-83. (1985). doi:10.1016/0196-9781(85)90036-1. PMID 2863809.
- ^ “Mechanism of action of L-leucyl-glycinamide and its effect on Parkinson's disease”. Advances in Neurology 45: 587-90. (1987). PMID 2881450.
- ^ “Modulation of agonist binding to human dopamine receptor subtypes by L-prolyl-L-leucyl-glycinamide and a peptidomimetic analog”. The Journal of Pharmacology and Experimental Therapeutics 315 (3): 1228-36. (December 2005). doi:10.1124/jpet.105.091256. PMID 16126839.
- ^ “Design and synthesis of photoaffinity-labeling ligands of the L-prolyl-L-leucylglycinamide binding site involved in the allosteric modulation of the dopamine receptor”. Journal of Medicinal Chemistry 49 (1): 307-17. (January 2006). doi:10.1021/jm050644n. PMC 2533518. PMID 16392815.
- ^ “Allosteric modulation of the dopamine receptor by conformationally constrained type VI beta-turn peptidomimetics of Pro-Leu-Gly-NH2”. Journal of Medicinal Chemistry 50 (26): 6725-9. (December 2007). doi:10.1021/jm070895r. PMC 2529021. PMID 18052024.
- ^ “Allosteric modulation of the dopamine D2 receptor by Pro-Leu-Gly-NH2 peptidomimetics constrained in either a polyproline II helix or a type II beta-turn conformation”. Journal of Medicinal Chemistry 52 (7): 2043-51. (April 2009). doi:10.1021/jm801575w. PMC 2712934. PMID 19271750.
- ^ “Specific binding of photoaffinity-labeling peptidomimetics of Pro-Leu-Gly-NH2 to the dopamine D2L receptor: evidence for the allosteric modulation of the dopamine receptor”. European Journal of Pharmacology 641 (2-3): 96-101. (September 2010). doi:10.1016/j.ejphar.2010.05.018. PMC 2907365. PMID 20639138.
- ^ “Inhibition by L-prolyl-L-leucyl-glycinamide (PLG) of alpha-melanocyte stimulating hormone release from hypothalamic slices”. Peptides 3 (6): 885-9. (1982). doi:10.1016/0196-9781(82)90055-9. PMID 6132363.
- ^ “Inhibition by MIF-I of alpha-MSH induced increase of intraocular pressure and miosis in rabbits”. Neuropeptides 12 (4): 213-7. (1988). doi:10.1016/0143-4179(88)90057-1. PMID 2907121.
- ^ “The effect of the blockade of alpha-melanocyte-stimulating hormone on LH release in the rat”. The Journal of Endocrinology 137 (2): 197-202. (May 1993). doi:10.1677/joe.0.1370197. PMID 8100849.
- ^ Sandyk R (May 1990). “MIF-induced augmentation of melatonin functions: possible relevance to mechanisms of action of MIF-1 in movement disorders”. The International Journal of Neuroscience 52 (1-2): 59-65. doi:10.3109/00207459008994244. PMID 1979968.
- ^ “MIF-1 is active in a chronic stress animal model of depression”. Pharmacology Biochemistry and Behavior 32 (3): 737-42. (March 1989). doi:10.1016/0091-3057(89)90027-0. PMID 2568001.
- ^ “MIF-1 potentiates the action of tricyclic antidepressants in an animal model of depression”. Peptides 12 (5): 915-8. (1991). doi:10.1016/0196-9781(91)90037-p. PMID 1686934.
- ^ “Improvement in major depression after low subcutaneous doses of MIF-1”. Journal of Affective Disorders 31 (4): 227-33. (August 1994). doi:10.1016/0165-0327(94)90098-1. PMID 7989637.
- ^ “Increased acquisition of a complex appetitive task after MSH and MIF”. Pharmacology Biochemistry and Behavior 3 (5): 901-4. (1975). doi:10.1016/0091-3057(75)90124-0. PMID 1801.
- ^ “Memory enhancement induced in chicks by L-prolyl-L-leucyl-glycinamide”. Pharmacology Biochemistry and Behavior 17 (5): 893-6. (November 1982). doi:10.1016/0091-3057(82)90467-1. PMID 6129646.
- ^ “MIF-1 can accelerate neuromotor, EEG and behavioral development in mice”. Peptides 11 (3): 527-32. (1990). doi:10.1016/0196-9781(90)90054-9. PMID 1974348.
- ^ “Brain Activation by Peptide Pro-Leu-Gly-NH(2) (MIF-1)”. International Journal of Peptides 2010: 1-10. (2010). doi:10.1155/2010/537639. PMC 2915805. PMID 20721355.
- ^ “Neurological effects of MIF-1, MSH, and opiate peptides in clinical studies”. International Journal of Neurology 14 (2-4): 205-9. (1980). PMID 6152908.
- ^ “Antiparkinsonian activity of L-propyl-L-leucyl-glycinamide or melanocyte-inhibiting factor in MPTP-treated common marmosets”. Movement Disorders 22 (5): 715-9. (April 2007). doi:10.1002/mds.21256. PMID 17373723.
- ^ “MIF-1 and its peptidomimetic analogs attenuate haloperidol-induced vacuous chewing movements and modulate apomorphine-induced rotational behavior in 6-hydroxydopamine-lesioned rats”. Peptides 28 (10): 2009-15. (October 2007). doi:10.1016/j.peptides.2007.07.026. PMID 17766011.
- ^ “Differential metabolism of Tyr-MIF-1 and MIF-1 in rat and human plasma”. Biochemical Pharmacology 47 (4): 699-709. (February 1994). doi:10.1016/0006-2952(94)90133-3. PMID 7907473.
- ^ “The Tyr-MIF-1 family of peptides”. Neuroscience and Biobehavioral Reviews 18 (4): 519-25. (1994). doi:10.1016/0149-7634(94)90005-1. PMID 7708364.
- ^ “Opposite direction of transport across the blood-brain barrier for Tyr-MIF-1 and MIF-1: comparison with morphine”. Peptides 15 (1): 23-9. (January 1994). doi:10.1016/0196-9781(94)90165-1. PMID 7912427.
- ^ “Melanocyte-stimulating hormone release-inhibiting factor-1 (MIF-1) can be formed from Tyr-MIF-1 in brain mitochondria but not in brain homogenate”. Journal of Neurochemistry 64 (4): 1855-9. (April 1995). doi:10.1046/j.1471-4159.1995.64041855.x. PMID 7891114.
- ^ “A potent and selective endogenous agonist for the mu-opiate receptor”. Nature 386 (6624): 499-502. (April 1997). Bibcode: 1997Natur.386..499Z. doi:10.1038/386499a0. PMID 9087409.
- ^ “From MIF-1 to endomorphin: the Tyr-MIF-1 family of peptides”. Peptides 28 (12): 2411-34. (December 2007). doi:10.1016/j.peptides.2007.10.006. PMID 17988762.
- ^ “Behavioral effects of neuropeptides in rodent models of depression and anxiety”. Peptides 31 (4): 736-56. (April 2010). doi:10.1016/j.peptides.2009.12.015. PMID 20026211.
- ^ “PAOPA, a potent analogue of Pro-Leu-glycinamide and allosteric modulator of the dopamine D2 receptor, prevents NMDA receptor antagonist (MK-801)-induced deficits in social interaction in the rat: implications for the treatment of negative symptoms in schizophrenia”. Schizophrenia Research 125 (1): 88-92. (January 2011). doi:10.1016/j.schres.2010.09.025. PMC 3010311. PMID 21036015.